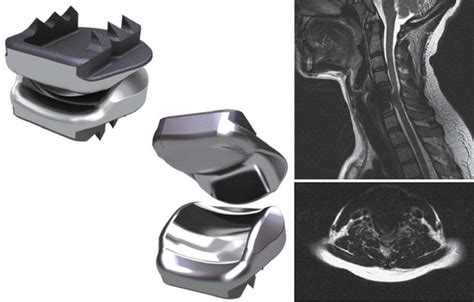
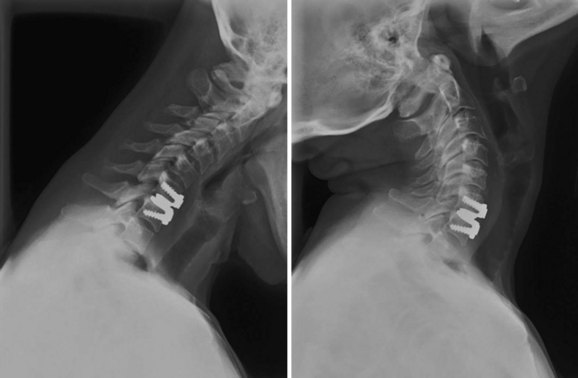
“Metallosis,” which is a buildup of metal debris from a medical implant, can cause all sorts of sickness and disorders. In the bloodstream, it triggers autoimmune reactions. In situ, it causes pseudotumors as the body works to encapsulate the foreign matter.
The Cover Up
Stryker had promised to keep the CerviCore study subjects safe and to keep them informed of any new information it learned about the product now in their necks.
But, the company knew of the manufacturing defects and said nothing.
As the CerviCore clinical trial progressed, adverse event reports surfaced that showed patients having metallosis reactions, but the company “adjudicated” each one as an isolated event not related to the device.
The FDA repeatedly raised concerns about the safety of metal-on-metal devices, but Stryker never passed these warnings on to the surgeons who were providing care to the 240 CerviCore recipients.
And, when Stryker gave up on trying to get CerviCore approved and applied for permission to end the clinical trial, the FDA allowed that only if Stryker warned doctors and patients about the potential metallosis danger, but Stryker never did that.
Once the CerviCore product no longer had the potential to make Stryker money, the company engaged in a full-scale cover-up. It aggressively told the implanting surgeons that their patients were imagining symptoms or that their symptoms were not related to the CerviCore device. All the while, Stryker knew the product was actually made defectively. The company even told the FDA, surgeons, and patients that it knew of no risk associated with the device even though it knew of the manufacturing errors and the sickness they are causing. Still to this day, Stryker has not come clean to the 240 people involved in the CerviCore clinical trial.